On October 23, 2025, China's General Administration of Customs (GACC) released its list of non-compliant imported food products for September 2025. A total of 443 batches from 45 countries and regions were denied entry, representing a month-on-month decrease of approximately 28% (down 174 batches from 617 in August) and a year-on-year decrease of about 6% (down 28 batches from 471 in September 2024).
Key Issues Identified:
The main problems were concentrated in the following areas: non-compliant labeling (16.5%), detection of melengestrol acetate (12.6%), discrepancies between goods and certificates (9.0%), registration issues with foreign manufacturers (7.0%), and failed sensory inspections (6.8%). Additional concerns included detection of animal diseases, furazolidone metabolites, sodium metabisulfite, parasites, and unauthorized animal-derived ingredients.
Brands Affected:
Well-known brands appearing on the list include Tyson (USA), House Foods (Japan), Coca-Cola (UK/Hong Kong), and Nestlé (Malaysia/Brazil/UK), among others, with specific batches recorded as non-compliant or not approved for import.
Notably, among the non-compliant import records in September, there were 12 cases involving new food ingredients. Of these, five batches of herbal tea from Kazakhstan were denied entry, with all cases citing the use of unapproved new food ingredients as the reason.
According to the Administrative Measure for the Safety Review of New Food Ingredients, a new food ingredient refers to any of the following items that do not have a traditional history of consumption* in China:
1. Animals, plants and microorganisms;
2. Sunstances isolated from plants, animals and microorganisms;
3. Food ingredients whose molecular structures have been modified;
4. Other newly innovated food ingredients.
*Traditional history of consumption: foods that have been produced and sold in fixed or non-fixed packaging in a provincial region for over 30 years and are not listed in the Chinese Pharmacopoeia.
New food ingredients must also:
Possess characteristics suitable for food use,
Meet nutritional and hygiene standards,
Be non-toxic and pose no short- or long-term health risks.
Note: Before 2007, China used the term "novel food", which was later replaced by "new food ingredients", representing the same regulatory concept at different stages.
According to the Regulations on the Submission and Acceptance of New Food Ingredients, the following do not qualify as novel food ingredients:
1. Items that do not have the characteristics of food materials;
2. Ingredients already listed in the national food safety standards, such as GB2760 (Standards for Food Additive Use) or GB14880 (Standards for Nutritional Fortifiers Use);
3. Ingredients for which the National Health Commission has issued a refusal of administrative approval;
4. Other items that fail to comply with relevant laws, regulations, or the requirements for novel food ingredient management.
To date, China's National Health Commission (NHC) has approved over 150 new food ingredients, including earlier "novel foods." Approved ingredients cover a wide range of sources, including plant-derived materials, probiotics, and functional components, and the number continues to grow. China NHC regularly publishes the latest approved lists in official announcements.
The public can also search the full list via the NHC's platform:
https://slps.cfsa.net.cn/xwfb/gzcx/PassFileQuery.jsp.
Applications for new food ingredients not yet approved must be submitted to China NHC. The process generally includes:
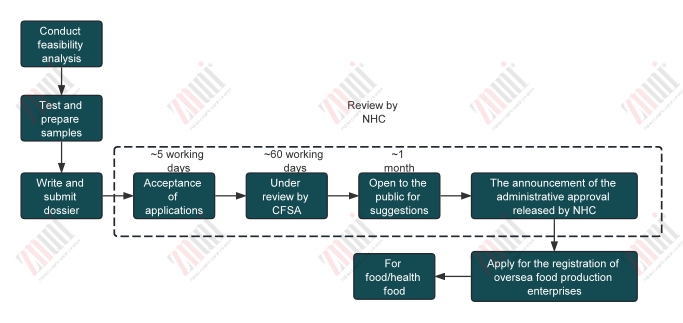
Required application materials typically include:
Application form
Development report
Safety assessment report
Production process
Relevant standards
Labeling documentation
Sample submission
ZMUni Compliance Centre reminds all industry stakeholders that food safety is of utmost importance. Companies are encouraged to strengthen pre-import compliance reviews, verify customs documentation carefully, and enhance supplier quality management to reduce the risk of import rejections.
Overseas food manufacturers and importers should also keep pace with the latest food laws and standards, and seek professional compliance advice when needed, to ensure smooth and timely market entry.
With our extensive expertise in pre-packaged food compliance and import clearance, ZMUni is committed to providing reliable, professional, and efficient support to help your products enter China and other key markets successfully. For inquiries, please contact us at info@zmuni.com.